Pioneering skin tag remover TagBand surpasses one million sales after device goes viral

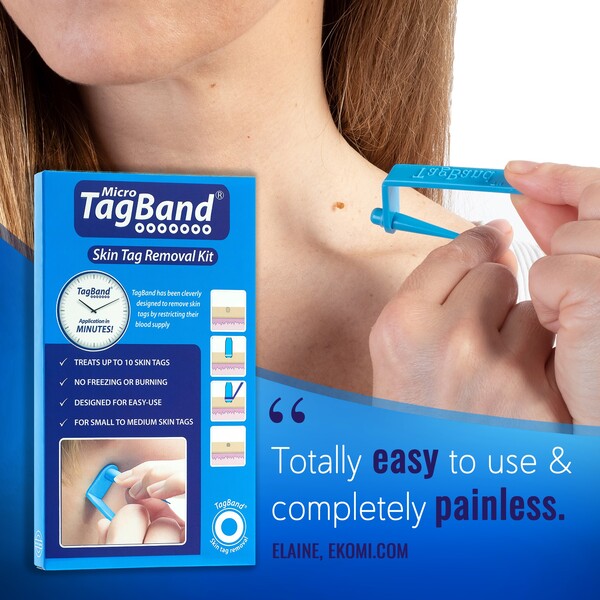
TagBand devices have been designed to remove skin tags via ligation – making a formerly troublesome treatment affordable, accessible and simple.
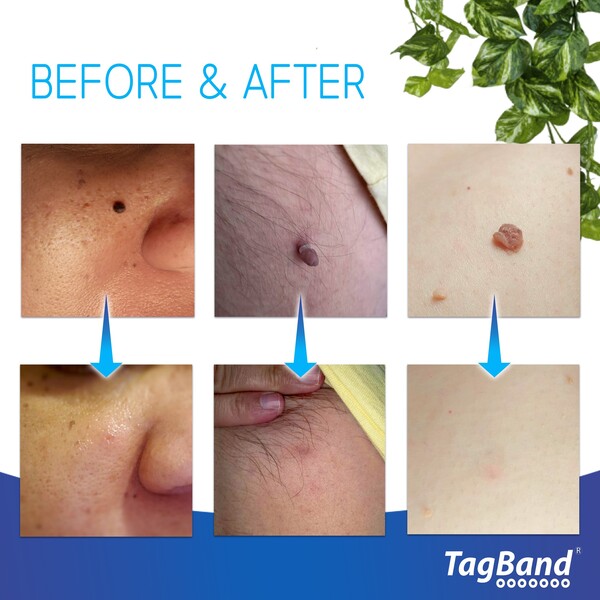
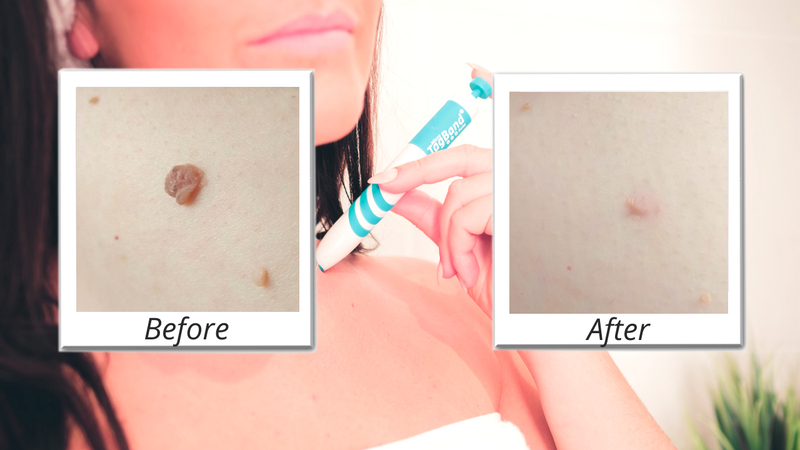
TagBand has surpassed one million sales after developing and popularizing a pioneering skin tag removal device.
The game-changing product utilizes the removal method known as ligation by applying silicone bands around the base of skin tags. Ligation means to restrict blood supply which is vital to a skin tag’s growth and survival.
With skin tag removal often not covered by health insurance policies, sufferers have even resorted to home methods – such as cutting off growths with nail scissors.
But TagBand devices have provided a non-invasive and budget-friendly solution to a prevalent health issue. Sales have hit seven figures since the brand launched in 2015 – cementing TagBands’ status as a market leader.
Devices come with 10 rubber bands included – capable of treating up to 10 separate skin tags.

Katie Harrison, General Manager of TagBand parent company UK Innovations GP Ltd, stated: “The TagBand range was born after a family friend of ours couldn’t afford surgery to remove her skin tags ahead of her wedding day.
“As there were no affordable home treatments available, she cut off her skin tags with scissors, causing a lot of pain and scarring.
“Skin tag removal isn’t covered by medical insurance and nearly 50% of Americans are affected by skin tags.
“Our mission is simple: ‘Improve the lives of consumers with the use of thoughtful innovation’.
“This is why we’re dedicated to creating problem-solving products that are accessible to all.”
TagBand is a subsidiary of UK Innovations GP Ltd, a retail business which originally launched in 2007.
The company currently performs most of its manufacturing in Britain, heading up two national offices in Northamptonshire and London.
All TagBand products are currently sold via Amazon in the USA. The full range is available on Prime delivery.
Visit the TagBand website for more information.
– Ends –
Contact
Phone: 0800 195 6633
Email: info@tagband.co.uk
Notes to Editors
TagBand is a brand operating under the umbrella of UK Innovations GP Ltd, based in the UK.
UK Innovations was founded in 2007.
TagBand was launched in 2014.