Health management company eNano Health develop a saliva test that could help the UK lift the lockdown
[ad_1]
Biomedical science company, eNano Health, have developed their unique test swab previously been used for viral infections such as SARs and MERS, in a response to the global pandemic. Unlike the current tests that look for antibodies or antigens, the saliva swabs measure changes that are present in the immune system, up to three days before symptoms even develop.
Bringing over 10 years of experience in monitoring illnesses and contagious viruses such as SARS, their non-invasive screening procedures that will meet the huge demand for rapid, regular testing during the Coronavirus pandemic. And with the news of several pubs having to close again shortly after re-opening for two days, the importance of testing to help control the virus and prevent further lockdowns is crucial.
Executive of the subsidiary company, eNano Health Europe, Peter Chan, says: ”Using eNano’s specific experience handling previous pandemics and outbreaks for countries in the Far East, we’re able to apply their extensive knowledge and experience to the current Coronavirus pandemic here in the UK. We’re confident the saliva swab is a cost-effective solution which will modernise the way in which the UK handles many other contagious viruses, moving forward.”
eNano Investor Tweedie Brown CBE adds; “Our main focus has always been to introduce a health management system in Europe. It so happened a pandemic broke out, and so we are able to offer an experienced pair of eyes offer assistance”.
As a localised lockdown is imposed on Leicester and some pubs swiftly shut again due to outbreaks, the saliva swabs would provide an effective solution to offering widespread testing quickly and efficiently. eNano Health’s swab could help instil confidence and help people get back to working and business reopening safely. The saliva swabs offer a cost-effective and easy to use solution that is scalable to supply the national coverage needed.
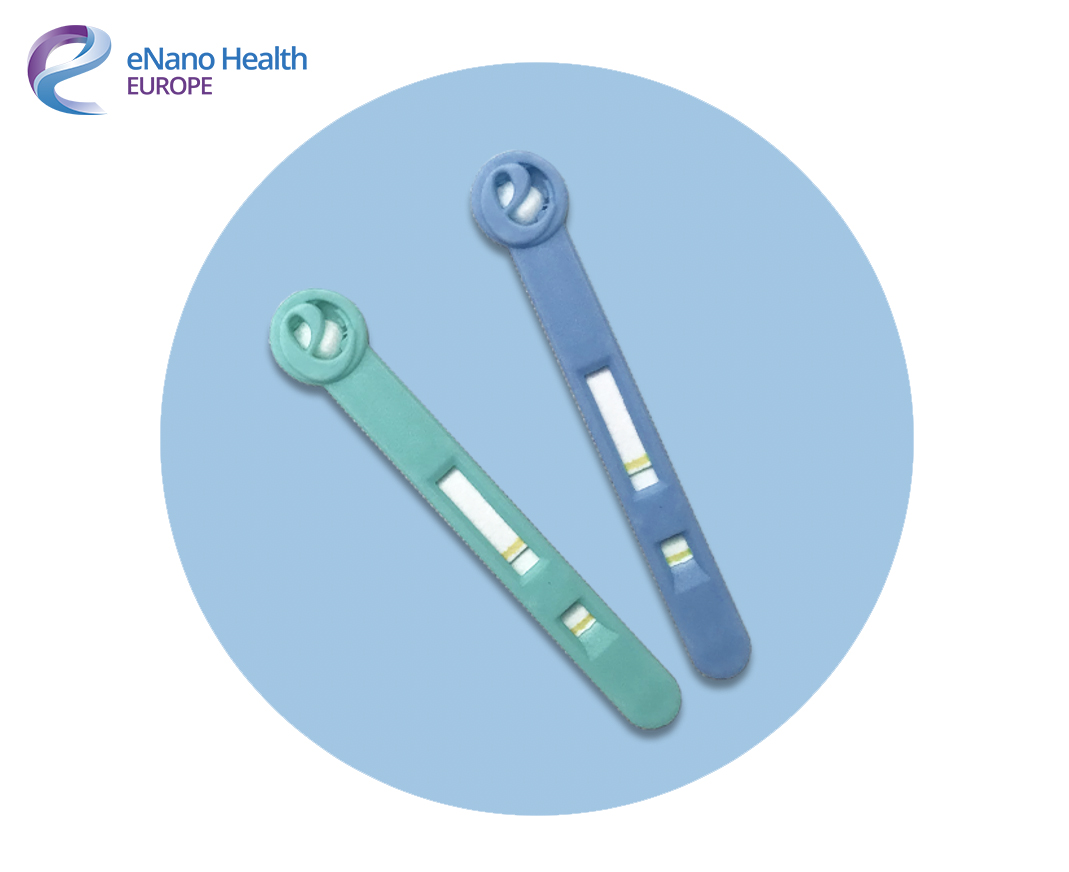
The swab tests already meet ISO 13485 standard and are now awaiting FDA, MHRA and CE accreditation for launch in Europe. Once granted, the next version of the test will change colour if a virus is present in the immune system- with or without symptoms being present.
These tests will not only significantly reduce the risk of false-negative results but also helps those who may have a virus such as Coronavirus but is asymptomatic, helping prevent the potential second wave. Further development means the swab will have a pregnancy test-style colour change, sharing results in a matter of minutes.
eNano Health and eNano Health Europe are now in discussion with leading Universities in the UK to start clinical trials for further sample pools for lab trials to process swabs before an official launch of the non-invasive home test kit alongside an accompanying app.
If you’ve had previous symptoms or want to put your pub or restaurant forward to take part in our lab trials, please email us on info@enanohealtheurope.com.
eNano Health Europe is also in discussions with a pilot scheme to open up one of London’s most popular tourist spots, Chinatown, safely using their saliva test kits allowing customers to visit restaurants.
For more information about the saliva test pilot scheme, visit https://enanohealtheurope.com/coronavirus/