- Achieving Excellence in Accounting: Lessons for Successful Professionals
- Kasra Dash Opens TaxBite Accountants Manchester – Lower Byrom St, Manchester M3 4AL
- CPA John Savignano on the 15 Traits Accountants Need To Succeed
- 360 ACCOUNTANTS ADDRESS HUGE FINANCIAL BURDEN FACING BUSINESSES
- Want to find the correct accountant for your business?
- YORKSHIRE ACCOUNTANTS ANNOUNCE NEW WEALTH MANAGEMENT PARTNERSHIP
Tosçelik Spiral Boru, an esteemed division of Tosyalı, Türkiye’s dominant …
Custom backgrounds for your office team are not difficult to …
Change is a constant in today’s dynamic business landscape, and …
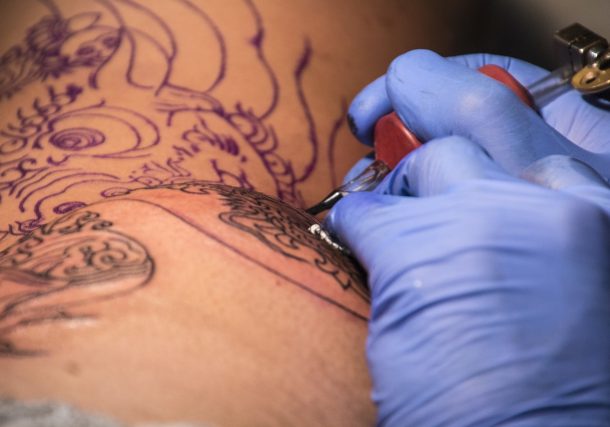
Amsterdam, 16-4-2024 – Tattoo culture has long been thriving in …
Technology
Finance
Business
Health
Lifestyle
Recent News
View AllIn the intricate world of lending, the concept of creditworthiness reigns supreme, serving as the cornerstone upon which …
Uncovering Value In the competitive world of private equity, success largely hinges on the ability to identify undervalued …
DigiDNA proudly presents iMazing 3 for macOS and Windows, the newest version of the premier iPhone manager in …
Have you ever wondered how products in your home are made so quickly and precisely? Robotics programming is …
ThinkOTB, a marketing agency based in Leeds, has been selected to assist Woolroom, a prominent wool bedding retailer …
In a world where credit rules supreme, having a high credit score is more vital than ever. Unfortunately, …